Search
Research
Safety of live attenuated herpes zoster vaccine in Australian adults 70-79 years of age: An observational study using active surveillanceTo assess the safety of live attenuated herpes zoster vaccine live (ZVL) through cumulative analysis of near real-time, participant-based active surveillance from Australia's AusVaxSafety system. ZVL was funded in Australia for adults aged 70 years from November 2016, with a time-limited catch up programme for those up to 79 years. This cohort study monitored safety in the first two programme years through active surveillance at 246 sentinel surveillance immunisation sites.
Research
Case Report: Neonatal Varicella Acquired From Maternal ZosterThe incidence of neonatal varicella has decreased dramatically since the introduction of the varicella vaccination. Although the varicella zoster virus is often associated with a mild infection, it may cause severe morbidity and mortality, particularly in the neonatal period and immunocompromised hosts. We report a case of neonatal varicella acquired from maternal zoster in a mother on biological immunosuppressive therapy.

News & Events
Four BrightSpark Fellowships awarded to early-career researchers at The KidsCongratulations to four outstanding early-career researchers from The Kids Research Institute Australia, who have been awarded BrightSpark Foundation fellowships and project funding for 2026.
News & Events
New cultural safety initiative guides best practice for clinical researchPerth investigators involved in a major global trial have launched an innovative Cultural Information Hub to maximise cultural safety for Aboriginal and/or Torres Strait Islander patients participating in research.

News & Events
Whooping Cough Day marks 10 years of vaccine advocacy efforts for Catherine Hughes AMWhooping Cough Day in 2025 has a special significance to Catherine Hughes AM and her family - it marks a decade of dedication to vaccine advocacy after the loss of their four-week-old baby son Riley in 2015.

News & Events
Announcing our 2025 Premier’s Science Awards finalistsEight outstanding researchers from The Kids Research Institute Australia and the Institute-led Broome STEM Festival are finalists in the 2025 Premier’s Science Awards.

News & Events
WA Government launches ‘game-changing’ nasal influenza vaccine program for kidsWestern Australian kids will have access to a needle-free nasal flu vaccine for the first time in 2026 as part of a new initiative to boost vaccination rates against the life-threatening virus.

News & Events
Latest Deborah Lehmann Research Award recipient tackles malaria in MadangPapua New Guinean researcher Dr Lincoln Timinao has been awarded the 2025 Deborah Lehmann Research Award (DLRA) for his work aimed at investigating the burden of malaria in young children.
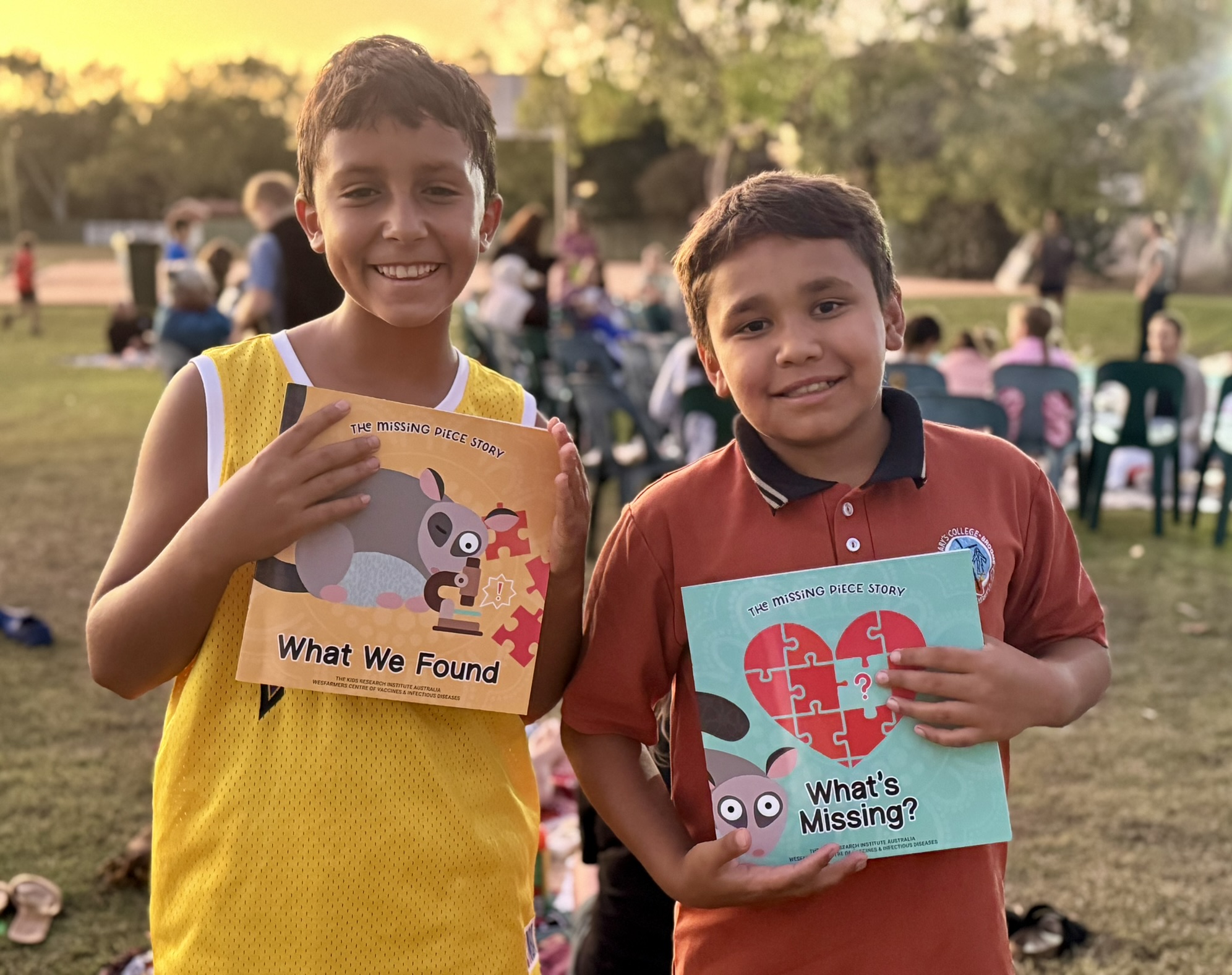
News & Events
Spectacular sunset launch for the Missing Piece Story BooksWesfarmers Centre of Vaccines and Infectious Diseases researchers Dr Janessa Pickering and Dr August Mikucki travelled to Broome last week for the official launch of the long-awaited Missing Piece story books.

News & Events
STEM festival is coming to Kalgoorlie this AugustFree Family-Friendly Science Fun During National Science Week 2025. Get ready for an awesome adventure into the world of Science, Technology, Engineering and Mathematics!
